Why does battery life get worse over time?
Your phone's battery isn't infallible, and you'll notice it the longer you use your phone.
Get the latest news from Android Central, your trusted companion in the world of Android
You are now subscribed
Your newsletter sign-up was successful
I'm sure you've noticed that you see a notable difference in how well a phone battery holds a charge after a year or so. If you keep a phone long enough, its battery may not even have enough charge to survive a whole day. Have you ever wondered why?
The answer is both simple and not so simple. But to fully understand why your phone's battery won't always last as long as it did when you first bought it, you first have to understand how batteries work. We'll try to break it down as simply as possible.
Batteries: How do they work?
Electricity isn't magical. In fact, it's a pretty boring subject for most of us and we only want it to be there when we need to use it. But to understand why your phone needs charged more now than it did when you first got it, you need to know a little bit about how a battery works. Don't worry, we're going to stick with the basics here.
Electricity, like any sort of energy, isn't a thing you can create. All the things we think of as "making" electricity are really only converting one form of energy into another, and a battery uses a chemical reaction (energy) to build an electrical charge that can be metered out over time. Different materials can be used to build this charge, and they will produce different results. In our phones, we use lithium-based batteries because they provide a decent level of output for a reasonable cost.
Having said that, companies are working on using different materials to build batteries. Some can provide more capacity, some have a longer lifespan, and others are fire-resistant. Things will change eventually when the costs of more "exotic" batteries go down.
The estimated life of a phone battery is just that — an estimate.
Inside a phone battery, you'll find three components that are important for what we're talking about: a negative electrode (called an anode and typically made of graphite), a positive electrode (called a cathode and made from a mix of lithium and other metals), and an electrolyte solution. The chemistry between these three things is simple at its base and is why they can be used to store energy.
When you apply a charge to the electrodes (from your charger), lithium ions are positively charged and are attracted to the negative electrode. When you pull a charge away from the battery, these lithium ions lose their positive charge and are no longer attracted to the negative electrode.
Get the latest news from Android Central, your trusted companion in the world of Android
The longer you draw the stored energy from a charged battery, the number of lithium ions that are no longer charged increases until there are just not enough of them left to produce any output, and the battery is dead. Plugging it into a charger resets this cycle.

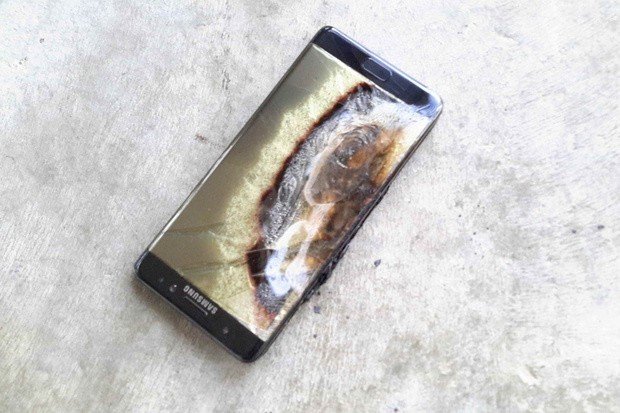
The electrolyte solution is 100% necessary, but it's also the most dangerous part of a battery. Because it's a liquid, it has a high heat capacity, and if all the heat it is holding reaches a certain point, it will rapidly expand or even explode.
If you notice your phone is swelling, stop using it right away and take it to a qualified technician.
When this excited electrolyte solution is exposed to the air, it can also catch fire. You may have seen videos of a battery "explosion" and know how violent it can be. Using your old, tired battery isn't inherently dangerous on the surface, but the loss of efficiency will lead to more heat, which could lead to... well...
The word "Cycle" is particularly important here. Because batteries are designed to store a charge, it's difficult to measure their usable life as a unit of time. A battery that lasts two years for you may only last six months for someone else because it's being used differently.
To estimate how long they are expected to last, battery longevity is measured by charging cycles. A phone battery is typically designed to last around 500 to 1,000 cycles, and a cycle is defined as charging a completely dead battery to 100% then draining it to zero again.
Charging a battery that has 50% charge left on it, then draining it back to 50% is a partial cycle, which is why you'll hear people telling you to charge your battery before it gets low and also hear people telling you the opposite as ways to game the system and stave off that 500th cycle. Of course, it doesn't work that way because the battery doesn't actually count the number of charge cycles. Five hundred is just an estimate.
But longevity can be measured in cycles because of what happens when you charge a battery and how it affects future charging cycles, the amount of energy that can be stored and the potential (think the number of volts) of the stored charge.
Oxidation and efficiency hate each other

Because electric vehicles are a real thing and the batteries they use are extremely expensive, numerous studies have been conducted to investigate why lithium-ion batteries degrade during their lifetime. Thankfully, this also applies to the less-expensive (but still expensive) batteries inside our phones, and it's because of chemical changes that occur during battery charging.
We know that charging a battery involves positively charging lithium ions, which are then attracted magnetically (electricity is a form of magnetism) to the negative electrode. As more and more charged ions are attracted, the difference in potential between the negative electrode and the positive electrode increases. That's how you measure voltage — the difference in potential energy between two electrodes.
Once it reaches a specific reading, the battery is considered fully charged. The opposite is true while discharging a battery, and the difference of potential decreases until it reaches zero because no more positively charged ions are present at the negative electrode. But that doesn't mean the negative electrode is clean and exactly the same as it was before you started.

Electrodes oxidize. The same way water and air can cause iron to rust (which is where the word oxidation comes from), lithium, graphite, and electrolyte salts will cause an electrode to oxidize. When every positively charged ion is stripped away from the anode in a battery, a microscopic layer of particles is left behind and has been chemically bonded to the graphite anode.
These particles are made from lithium oxide (lithium bonded with oxygen) atoms and lithium carbonate (lithium bonded with carbon) atoms, neither of which has the same chemical or electrical properties as graphite. This layer interferes with the charge/discharge cycle, and both the difference of potential (voltage) and the number of charged ions that can be attracted change.
Eventually, the changes are enough to notice. If you continue to use the battery and charge it as you normally would, you reach the point where there isn't enough electrical energy being stored to power your phone.
Charging a battery essentially changes the composition of the electrodes and affects the way it will charge in the future.
Different types of lithium compositions, as well as various salts used in the electrolyte solution, affect the amount of these deposits left behind on the electrode. However, the materials that facilitate a cleaner cycle aren't necessarily the best because they can't provide as much stored power.
We want high-capacity, low-power batteries in our phones because they are safer than high-power batteries (and cost less), and we want them to provide power to our phones as long as they can. An electric vehicle can use high-capacity, high-power batteries because a solid frame protects them, and they aren't as likely to be damaged. They're necessary because a car needs to be able to go long distances between charges.
However, the cost of a replacement battery for a Tesla Model S is $12,000, too. Part of that cost comes from the expensive materials used to build a lithium-nickel-cobalt-aluminum-oxide battery as opposed to the basic lithium-cobalt batteries used in a phone that don't last nearly as many cycles before they degrade.
Voltage matters

One of the primary factors that can impact the lifespan of a lithium-ion battery is its voltage. Phones and cars aren't the only things designed to run on rechargeable lithium batteries.
In 2015, the U.S. Department of Energy invested a significant amount of money and time to identify the causes of problems and develop mitigation strategies, as satellites utilize lithium-based batteries and solar chargers. Studies found that after the composition of the battery itself, the next biggest culprit that can affect battery longevity is the charging voltage and the voltage of the held charge.
The chemistry that makes a lithium battery work naturally degrades the anode, which is what we discussed above. However, if you charge a battery with more than 3.9 volts or store a charge with a potential difference higher than 3.9 volts, the same sort of degradation occurs to the cathode (positive electrode). This essentially cuts the battery's longevity in half.
Charging voltage and held voltage are essentially the same thing because you're exciting all the components of a battery, but charging also introduces heat, and the higher the charging voltage, the hotter it will be. Heat applied when a battery is excited higher than 3.9 volts further worsens the degradation of the cathode.
There is no secret cabal of battery makers who are trying to fleece us; it's all chemistry.
In other words, the voltages necessary to power a modern phone and quickly charge its battery mean it's almost impossible to "fix" things. Anyone with a battery-powered drill has seen this in action. The 12 or 14-volt batteries used in a tool don't last nearly as many cycles as the ones in our phones. They store and operate at a higher voltage, charge at a higher voltage and at a much hotter temperature, and can be noticeably affected after just a few charging cycles.
They use the same basic lithium-based batteries as a phone because using the sorts of materials we see in a Tesla S battery would make them more expensive, and they just don't have a very long lifetime. Thank goodness we can recycle most of the materials in them, and we're not drowning in a sea of discarded Makita and Porter-Cable batteries with lithium being more expensive than gold.
The good news is that all the companies that manufacture lithium batteries are working on improving their products. The first company to develop a battery that lasts significantly longer will likely generate substantial profits from it. All we can do is charge our phones when they need to be charged, and know that there isn't some conspiracy between battery manufacturers to get us to buy new products more often.

Jerry is an amateur woodworker and struggling shade tree mechanic. There's nothing he can't take apart, but many things he can't reassemble. You'll find him writing and speaking his loud opinion on Android Central and occasionally on Threads.
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.
